How the 2025 Nobel Prize Helped Shaped MS Research
Oct 19, 2025If you follow science news (or even if you don’t!), you may have heard about the new 2025 Nobel Prize in Medicine and Physiology — awarded to three scientists who uncovered how our immune system learns not to attack our own bodies.
And if you live with an autoimmune condition like multiple sclerosis (MS), you may have wondered: “Have their discoveries affected me?”
In this post, we’re going to explore exactly that.
But first, we need to backtrack and understand a bit more about how the immune system works.
Our Immune System Soldiers and the “Boot Camp”
You can think of your body as a civilisation of cells. A country that needs protecting. Every day, it faces intruders — from bacteria, viruses, or even rogue cells of your own that have gone off script.
To defend against these threats, the body relies on the adaptive immune system, which is the organised system of white blood cells that functions like our bodies’ military. It has the ability to recognise intruders, memorise how they look, and importantly how to distinguish them from civilians (our own cells).
One of the key branches of this system is made up of T cells — the foot soldiers of immunity.
T-cells: the Immune System’s Soldiers
When one of our cells becomes infected with a virus or other foreign invader, it displays small fragments of that invader on its surface using special molecules from the human leukocyte antigen (HLA) system.
One kind of T-cells, called helper T-cells (CD4⁺), patrol the body and constantly scan these HLA molecules. If they detect a fragment of a foreign invader (called an antigen), they recruit cytotoxic T cells (CD8⁺) to directly destroy infected or abnormal cells using special chemical weapons.
Here’s the important part though. This only works because each T-cell only has a specific antigen receptor, like a scanner tuned to recognise one specific target only. Our body creates such a gigantic number of different variations (10^15 in fact, that’s 10 with 15 zeros behind it) in these antigen receptors for T-cells, with the idea being that no matter what kind of foreign invader enters our body, a T-cell will be there to recognise it.
And when an invader is recognised, the T cells rapidly clone themselves to create an army of identical defenders ready to fight that specific threat.
T-cell Boot Camp: Learning Friend from Foe
But, because the body generates such an insane number of unique T-cell receptors, there’s always a risk that some will mistakenly target the proteins on our own body’s cells. So to prevent this “friendly fire,” every new T-cell must first pass through rigorous training in an organ called the thymus.
Inside the thymus, developing T-cells are screened to see whether they recognise our own bodies as a threat. If they do, they’re removed before they even enter the bloodstream.
The result is a refined army of T-cells that can respond to genuine threats while leaving the body’s own tissues unharmed — a process known as central tolerance.
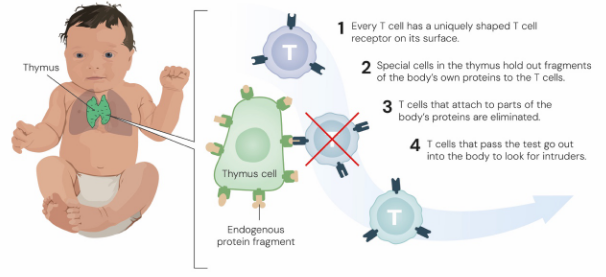
Image retried from: https://www.nobelprize.org/uploads/2025/10/popular-medicineprize2025-2.pdf
… at least in theory. In practice, some self-reactive cells inevitably slip through training. It seemed like the body had a second line of quality assurance, to ensure these rogue T-cells don’t become a problem.
But for a long time we didn’t know how this second line of defence worked. And that’s where our Nobel prize winners come in.
The 2025 Nobel Prize
If the thymus is our T-cell boot camp, then the next step in this story is discovering the immune system police — the special agents that stop mis-trained soldiers from going rogue.
Discovery 1: The discovery of “regulatory” T-cells
In the mid-1990s, Japanese immunologist Shimon Sakaguchi made a landmark observation. When he removed a small subset of helper T cells marked by the molecule CD25, mice developed severe autoimmune disease. When he restored those cells, the inflammation subsided (Sakaguchi et al., 1995).
This showed that a tiny population of T cells weren’t attackers at all — they were suppressors, actively keeping the immune system in check. These cells were later renamed regulatory T cells (Tregs) to reflect their peacekeeping role.
Discovery 2: The FOXP3 gene
A few years later, Mary Brunkow and Frederick Ramsdell were studying a mysterious autoimmune syndrome in mice called scurfy — and its human equivalent, IPEX syndrome. Eventually, they made the next big leap, finding that the gene FOXP3 was essential for the development and function of these regulatory T cells (Bennett et al., 2001).
Without FOXP3, the body produced normal numbers of T-cells, but they lacked the ability to restrain immune responses. The result was unchecked inflammation and lethal autoimmunity. FOXP3 became recognised as the master regulator gene of the Treg lineage — the defining feature that turns an ordinary T-cell into a calm, disciplined peacekeeper that patrols the body for rogue soldiers.
Peripheral tolerance in a nutshell
Together, these Nobel Prize-winning discoveries laid the foundation for what scientists now call peripheral tolerance — the process that keeps the immune system balanced once its cells have left the thymus. While central tolerance removes dangerous recruits during training, peripheral tolerance is the oversight that happens in the field: Tregs (still trained in the thymus) patrol the bloodstream, recognise excessive or misdirected immune responses, and put a stop to them before damage happens.
This concept reshaped how we understand autoimmune diseases like multiple sclerosis, type 1 diabetes, and rheumatoid arthritis. Instead of viewing them purely as “overactive” immune conditions, researchers began to see them as a failure of immune regulation — when for whatever reason, this balance between offense and peacekeeping is unbalanced.
Impact on MS - It’s Complicated.
Regulatory T-Cells in MS
According to the most recent review we could find, it does appear that regulatory T-cell dysfunction plays a role in MS (Calahorra et al., 2022).
People with MS often show fewer Tregs expressing the FoxP3 protein (coded by the gene of the same name) and is essential for their regulatory function. Lower FoxP3 expression means these cells are less effective at calming overactive immune responses and may struggle to keep inflammatory cells under control.
Some studies have even reported that Tregs in people with MS can lose their suppressive abilities during periods of active disease, due to lost or weakened FOXP3 expression. This leaves the aggressive soldiers to then spread unchecked.
Why could this be? Well…
Genes Set the Stage — But Don’t Tell the Whole Story
Like most autoimmune diseases, the genetics of MS are complicated. No single gene “causes” MS, as far as we currently know..
Researchers have identified over 200 genetic markers linked to MS susceptibility, most of them involved in immune regulation (Baranzini et al., 2017). Yet, FOXP3 itself does not appear to be one of the main risk genes. Instead, the HLA-DRB1*15:01 allele stands out as the strongest and most consistent association. This variation of the gene causes a change to the human leukocyte antigen (HLA) system that underpins the “scanning” system that T-cells use, that we mentioned earlier.
Even then though, genetics alone cannot explain MS. Environmental and viral triggers — such as low vitamin D levels and Epstein–Barr virus infection — are also believed to act as the sparks that ignite disease in genetically susceptible individuals. We explore these risk factors further in our earlier post, What Causes Multiple Sclerosis?
Not Just T Cells
To add even more complexity, while T cells are major players in MS, they are not the only ones contributing to the damage.
In addition to them, the disease involves multiple immune cell types going rogue, including:
- B cells, which can produce harmful antibodies and help activate T cells
- Macrophages and microglia, which end up amplifying inflammation instead of cleaning up waste; and
Together, these findings suggest that MS is not driven by a single rogue cell type, but rather by a system-wide imbalance in immune regulation — where several layers of the body’s tolerance mechanisms fail at once.
Shaping the Future of MS Treatment
But despite all that complexity, this new understanding of Regulatory T cells have transformed how scientists are now approaching MS treatments. Instead of simply suppressing the immune system, new research aims to rebuild immune tolerance — helping the body relearn what it should and shouldn’t attack.
The discoveries that earned this year’s Nobel Prize are now transforming how scientists approach multiple sclerosis. Instead of broadly suppressing the immune system, new treatments are focused on rebuilding immune tolerance — helping the body relearn what it should and shouldn’t attack.
Early clinical work has explored Treg cell infusions, where regulatory T cells are extracted, grown in the laboratory, and then reinfused back into patients. These studies have shown that such cells can safely reach the central nervous system and may help stabilise disease activity in relapsing–remitting MS (Verreycken et al., 2022).
In parallel, researchers are developing engineered antigen-specific Tregs — immune cells designed to recognise myelin-related proteins and selectively suppress inflammation in the brain while leaving the rest of the immune system intact (Zhong & Stauss, 2024).
These are just examples, but show promising results!
However it’s important to remember that these emerging treatments are still experimental and not available outside research settings. We do recommend raising these topics with your treating neurologist though, so they can help keep you informed as new information arises!
And in the meantime, alongside disease-modifying therapies, exercise-based rehabilitation remains one of the most effective, evidence-backed ways to protect function and well-being.
If you’d like to delve into practical evidence-based strategies to help manage MS symptoms from home, we’d love for you to check out our MS PhysiKit!
References
2025 Nobel Prize Press Release. Retrieved October 2025 from: https://www.nobelprize.org/prizes/medicine/2025/press-release/
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M., & Toda, M. (1995). Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of immunology (Baltimore, Md.: 1950), 155(3), 1151-1164.
Bennett, C. L., Christie, J., Ramsdell, F., Brunkow, M. E., Ferguson, P. J., Whitesell, L., ... & Ochs, H. D. (2001). The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nature genetics, 27(1), 20-21.
Calahorra, L., Camacho-Toledano, C., Serrano-Regal, M. P., Ortega, M. C., & Clemente, D. (2022). Regulatory cells in multiple sclerosis: From blood to brain. Biomedicines, 10(2), 335. https://doi.org/10.3390/biomedicines10020335
Baranzini, S. E., & Oksenberg, J. R. (2017). The genetics of multiple sclerosis: from 0 to 200 in 50 years. Trends in genetics, 33(12), 960-970. https://doi.org/10.1016/j.tig.2017.09.004
Verreycken, J., Baeten, P., & Broux, B. (2022). Regulatory T cell therapy for multiple sclerosis: Breaching (blood-brain) barriers. Human vaccines & immunotherapeutics, 18(7), 2153534. https://doi.org/10.1080/21645515.2022.2153534
Zhong, Y., & Stauss, H. J. (2024). Targeted therapy of multiple sclerosis: a case for antigen-specific Tregs. Cells, 13(10), 797. https://doi.org/10.3390/cells13100797
Stay Informed!
News, updates, and science delivered to your inbox.
We will never sell your information, for any reason.

